Microbiological
Reporting
System
Version 3.0
The Future of
Environmental Monitoring
The Microbiological Reporting System (MRS) is a complete solution for the routine environmental monitoring and general management of Quality Control Laboratories and the Pharmacy Units that they serve.
Used and trusted by NHS Pharmacy and Regional Quality Control Labs in addition to many private healthcare providers throughout the UK, the system allows for the rapid and accurate entry of environmental monitoring data which can then be analysed and trended.
MRS Version 3 released in early 2021, replaces the longstanding MRS 2 which has seen over a decade of use. Version 3 brings a whole host of additional features to the system, whilst leveraging modern technologies and computing techniques for improved efficiency, ease-of-use, and performance.
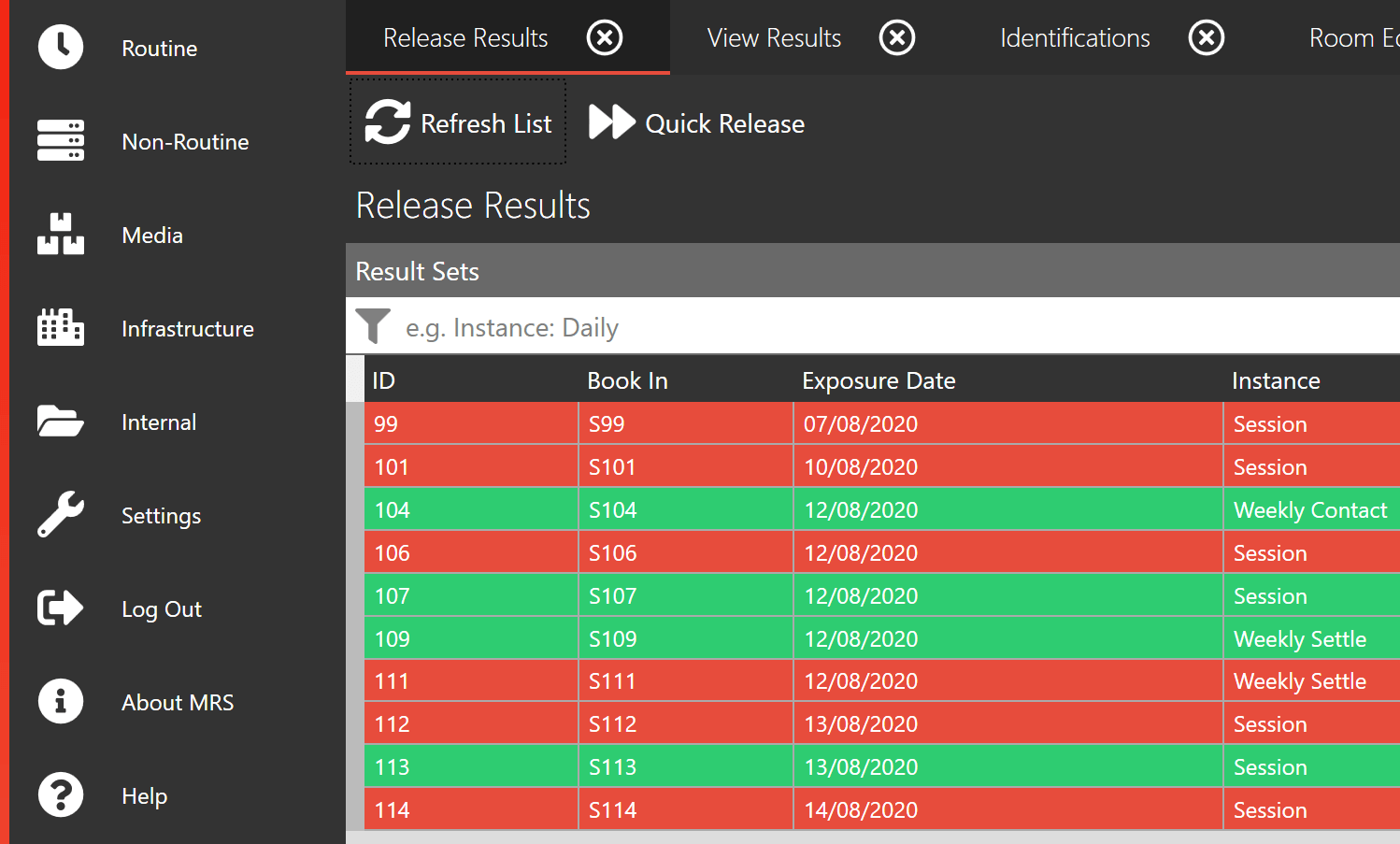
Rapid and Accurate Data Entry
MRS harnesses the power of both Barcodes and QR codes to facilitate rapid and accurate data entry, resulting in significant time savings and minimizing the potential for human error.
Seamless Lab and Customer Integration
Through the fully integrated web interface of QC Lab, customers have the convenience of accessing detailed information about their submitted plates. This user-friendly interface allows instant notifications of any exceptional results, ensuring prompt awareness of critical information. Additionally, customers can take advantage of a comprehensive reporting system within the interface, enabling them to identify potential issues and effortlessly track historical data. This robust system facilitates proactive identification of trends and supports efficient decision-making.
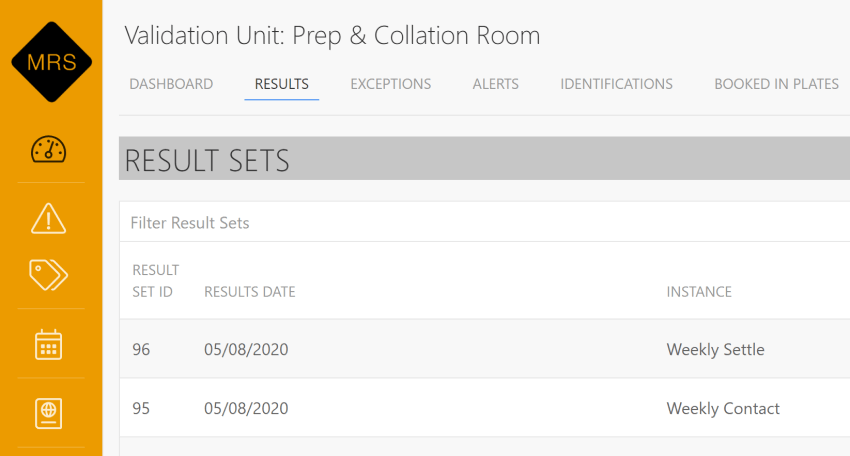
Next Level Reporting
With our extensive repository of historic data, you can leverage powerful analysis tools to identify recurring issues and trends within your laboratory. Our suite of fully customisable reports covers all aspects of your lab operations, providing you with comprehensive insights into your processes.
Manage Your Lab
Organise everything from within MRS: Chemical Inventories, External Services, Media Products, Internal Documentation and more!
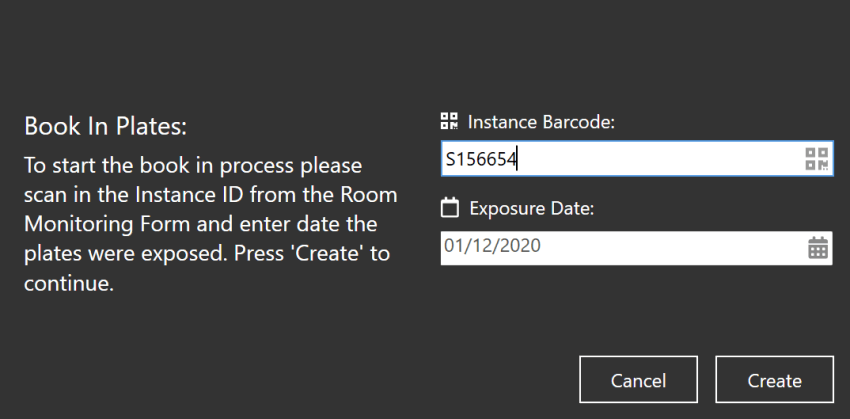
Web Book-in
The introduction of self-booking for units is a valuable feature within MRS Web. By allowing units to book in plates themselves, it streamlines the plate booking process, and improves data accuracy. This capability contributes to efficient laboratory operations, enhances communication between units and the lab, and reduces potential errors and delays.
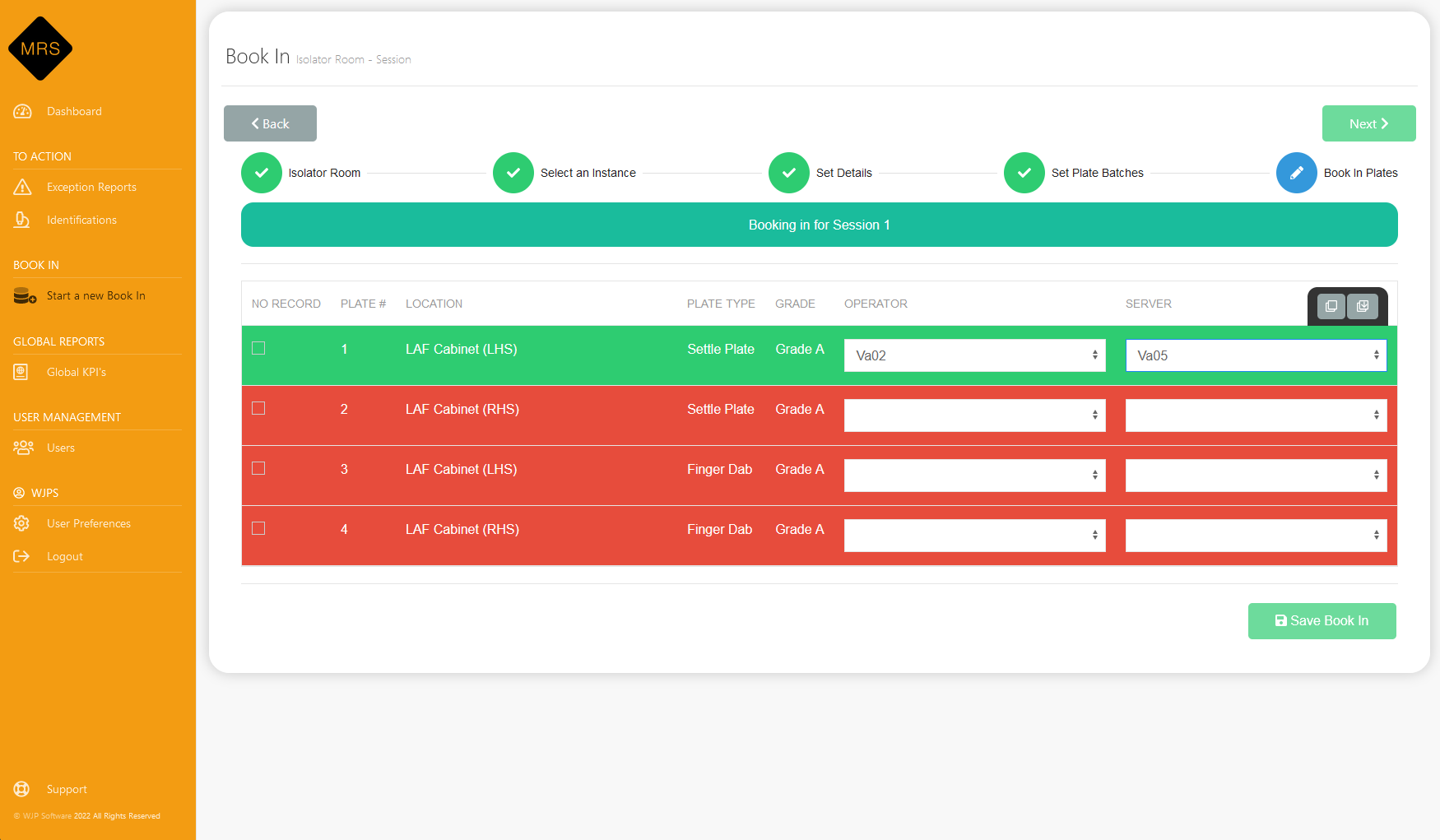
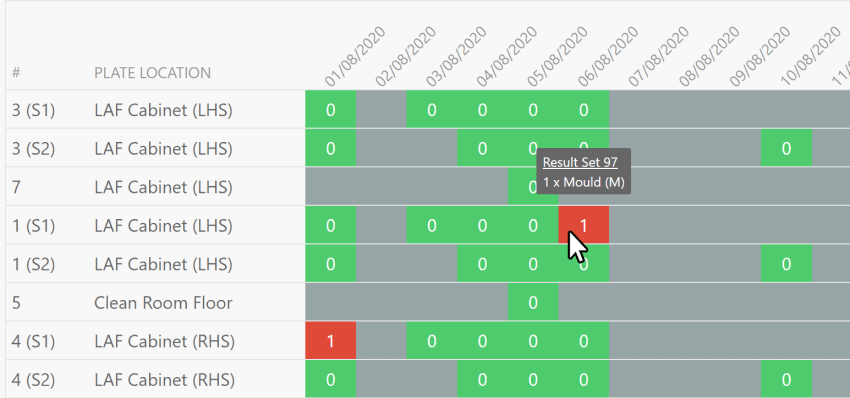
Calculate Alert Levels
MRS uses the standard deviation calculation in the quality assurance of aseptic preparation services based on previous results in the system.
You Lead The Way
The development of MRS is hugely influenced by our customers, with both regional and national user-groups taking place regularly. These contain sneak-peeks of upcoming features, useful tips and guidance through different aspects of the system, and are a chance to give your input on any changes you would like to see.


Priority Support
Benefit from truly world-class customer support. All of our support agents are intimately familiar with the software’s ins-and-outs, and the majority of queries are resolved in

Catch All Errors
A robust, time-proven workflow, with the addition of multiple checking stages severely limits the capacity for data errors to escape unnoticed.

Fully Compliant
MRS has been designed to fully comply with both GMP and MHRA standards and best practices.
Stockton Quality Control Laboratory (SQCL) have been working with WJPS since 2009. Working in conjunction with them we have added many new features required by a regional QC laboratory. In 2011 SQCL rolled out the system across their laboratory to manage 35 sites.
During this time WJPS has also worked with SQCL to develop a web based reporting system which allows end users to view their results online as soon as they have been processes. It also gives end users the power to generate their own trends.
“Using MRS allows our customers to continually comply with current Good Manufacturing Practice along with standardised reporting, and being able to create quality benchmarks.”

Bradford Royal Infirmary (BRI) turned to WJPS for expertise in helping convert a paper based system into a modern sophisticated solution.
Working closely together for 18 months MRS was developed. Originally going live in 2008 BRI have been incredibly pleased with the software which has saved them hours of data entry and helped improve their process and efficiency.
BRI are now able to retrieve up to date accurate information within second rather than the hours it used to take.
“Working with WJPS has been an invaluable experience. We would recommend MRS to any hospital needing a modern and visually appealing reporting system. MRS is continually evolving so it will always be at the forefront of microbiological reporting databases.”

Leeds Teaching Hospital (LTH) were one of the first Trusts to join us who were already using an existing application to record their environmental monitoring data, rather than an in-house approach. As one of the largest teaching hospitals in the UK, having a modern system that improves process and trending was key.
As part of the implementation we developed a bespoke add-on to help import species level identification information from the pathology system. This process helped remove any manual intervention required to update the results.
As a recent addition to the WJPS customer base, LTH have progressed from an installation, through validation processes and are now using the system in the live environment. The Trust are taking advantage of the Web Reporting interface to reduce the amount of paperwork created, and excess time spent creating reports.
“The MRS system we use has had to be configured to cope with a variety of situations, including licensed batch based areas and unlicensed aseptic and radiopharmacy areas. The installation went smoothly and the initial validation phase was supported by the comprehensive validation scripts provided by WJPS. Overall, our experience of the system has been positive and we have found WJPS to be very flexible and responsive to our needs.”

| Feature | MRS 2.5 | MRS 3.0 |
|---|---|---|
| Room management and Plate grouping | ||
| Generate standardised, barcoded room-monitoring forms | ||
| Quick and Accurate Plate Book-In Process | ||
| Enter Results Module | ||
| Exception and Alert report generation | ||
| Pre-written Standard Comments for Exception Reports | ||
| Trending and Reports | ||
| Permissions based User Management | ||
| Real-time Reporting for QC Lab Customers via MRS Web | ||
| Respond to Exception Reports online | ||
| Improved and more intuitive User Interface | ||
| Support for Grades attached to Plates | ||
| Room versions with modifiable alert limits | ||
| QR Code Support | ||
| Single failure exception and alert reports | ||
| Streamlined release process | ||
| Import and Export Plates | ||
| Room Audit | ||
| GMP compliant ‘Request for Analysis’ forms | ||
| Send and Recieve Identification to MRS Lab or via email | ||
| Lab Management functionality (Chemical Inventory, External Services, Inspection Dates, Lab Logs .etc) | ||
| No merged Result Sets | ||
| Unavailable | Contact Sales |
Make routine environmental monitoring easy.
Contact us for pricing, demonstrations, or advice on how MRS could be used in your lab set up.
Request a Demo